Digital Adherence Technologies (DATs) support patients around the world and across many different health areas to keep them adherent en successfully complete their treatment.
In high income countries (HICs), DATs are deployed to support clinical research trials, for medication that must be taken on a daily basis such as contraceptives, and for seniors who may struggle to remember to take their medications. In low- and middle-income countries (LMICs), DATs are more often used to target specific disease areas such as HIV and tuberculosis (TB).
These markets are often treated as fully distinct, making it difficult to identify promising technologies that could be used for purposes they weren’t explicitly designed for.
The 2021 Market Landscape for digital adherence technologies takes a wide view of the DAT market to develop a clear picture of the DATs currently in use across geographies and disease areas to evaluate the current ability of the overall DAT market to serve TB and to identify market trends and promising technology areas.
Click the icons to jump to the relevant section:
Conclusions & Market Outlook
Few existing DATs were explicitly designed to target users in middle-or low-income markets to support treatment of infectious diseases.
- Many of the DATs on the market are geared towards clinical trials applications or working directly with specialty pharmacies or home care agencies in high-income settings.
- Notable exceptions of manufacturers in South Africa and India suggests that there is more room to grow the market in LMICs.
A surprising number of DATs designed for high income markets are technologically compatible and affordable for LMICs contexts.
- A number of “high income” DATs were intentionally designed to be low-tech to support elderly populations who have trouble managing their medications.
- Similar prices among some DATs marketed for high income countries and LMICs suggests opportunities to bring down costs as the technologies proliferate and scale is realized.
A big challenge in adopting several DATs is that they are not designed for the blistered packaging used for TB.
- Despite this challenge, some manufacturers reported an interest in adapting their pill bottle technology to other container types.
Sustainability in the future will depend on developing and identifying products that can serve both the TB market as well as other health markets.
- Products that can bridge the gap between TB and broader use cases point out one potential direction for the market.
- Specific products covered in this assessment are designed for blister packs beyond TB indications such as birth control, while others can accommodate a wide range of medications.
- These products have multiple paths to scale, making them a potentially appealing option for the TB market.
Digital Adherence Technologies evaluated
A total of 19 DATs were evaluated, representing eight different types of DATs and target markets with varying income levels.
All DATs were evaluated based on two categories, which together comprise a DAT product’s ability to serve the TB market in LMICs
- Fit for Purpose is focused on the conditions a DAT will be used within, including factors such as human and technological requirements. Is the DAT well-suited to environmental considerations often characteristic of LMICs?
- Technical Specifications relates to the product design elements of the DAT. Does the product have the necessary technical qualities to serve its purpose?
Fit for purpose
Technical specifications
1. Ability to serve the TB market in LMICs
A product’s ability to serve the TB market in low- to middle-income countries is predicated upon its technical qualities and characteristics (technical specifications) and capacity to serve TB-affected individuals in LMICs (fit for purpose).
Most products score highly across Technical Specifications categories, suggesting that a high number of DATs meet the requisite technical features to function effectively for adherence support.
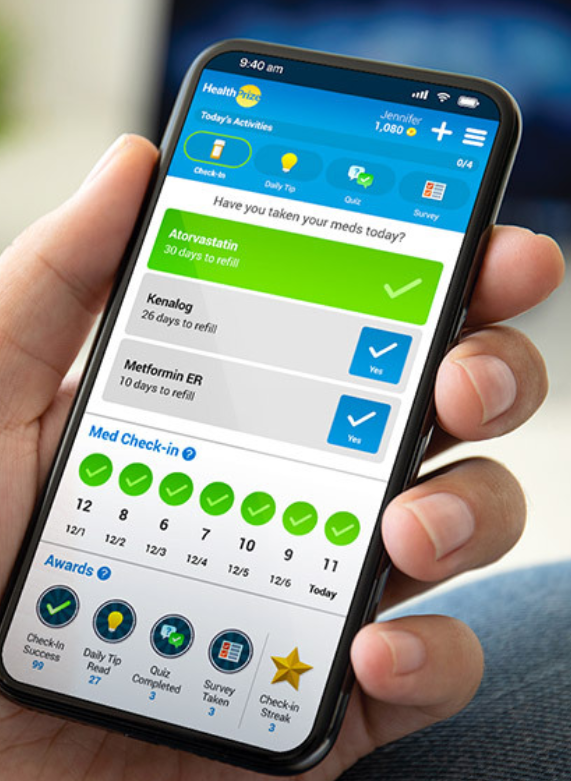
There is significant scoring variability across Fit for Purpose categories. However, even a low-scoring DAT may still have a role to play in TB support for LMICs. HealthPrize, the lowest scoring product in the assessment, is not designed to track adherence, but rather employs behavioral economics principles to encourage adherence. Elements of this program could are possibly useful for patients who struggle to maintain motivation through the latter stages of their TB treatment.
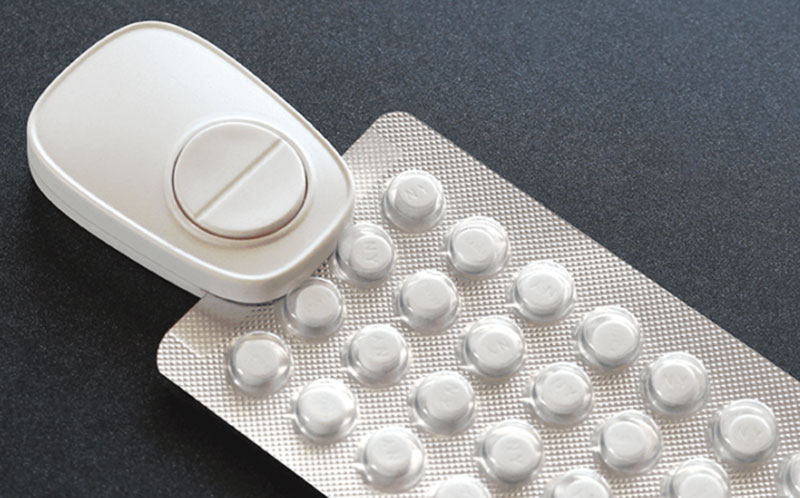
Similarly, simply because a product scores highly, does not mean that it can immediately serve the TB market in LMICs. For instance, a container design change would be needed to make the Smart Pill Bottle products effective for TB drug packaging.
2. Current gaps in the DAT market
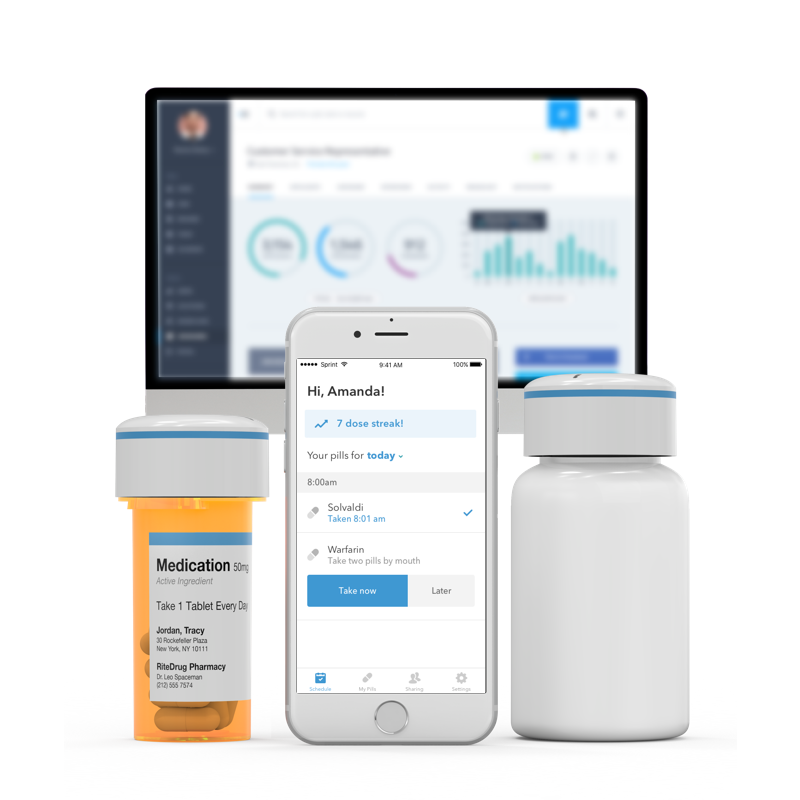
A deeper dive into the assessment categories reveals that it’s challenging – perhaps impossible – to create a DAT product that “does it all” for TB in LMICs. For example, there’s a tradeoff among some DATs between provider and patient burden for utilizing the product. Automatic pill dispensers such as CareDose and TMEAD have the lowest patient burden of any products evaluated, but require significant up-front preparation.
Certain product types have inherent limitations. For instance, several DAT products such as Smart Pill Bottles, a popular option in high income countries, are not well-equipped to TB medication in blister packs or complex regimens for drug resistant TB. While these products are not immediately ready to serve the TB market, the core technology may be transferable to different container shapes.
Some otherwise well-performing products have never been used for TB treatment or in LMICs previously. This doesn’t mean they’re not potentially useful. As the market for DATs continues to evolve globally, products like Popit could expand to serve markets for which they were not originally designed.
3. DAT design considerations for lower middle-income countries
Access to mobile technologies and electricity remain limited and unequal across many LMICs. As such, these factors are major design considerations for DATs in LMICs. In the tables below, DATs are characterized according to whether they require a smart or feature phone and the frequency that DATs require charging.
Many DATs that are not designed for TB or for LMICs still have low technological requirements. The market for DATs outside of LMICs includes a sizeable elderly population, who require support to maintain adherence to their regular medications. For this reason, DATs such as CleverCap and Adhere Tech do not require even a basic phone, even though they’re marketed for high income countries.
Emerging products offer Bluetooth solutions that are low-tech and patient-centric. CuePath turns blister packs into adherence devices by applying an electronic sticker to their back. When a patient expresses their medication, a signal is sent via Bluetooth to a gateway, which transmits the data via mobile connection.
4. Affordability
Affordability is challenging to measure across the DAT market because the range of offerings is vast, from digital-only products such as Video Observed Therapy, to physical products with a digital component, and a diverse set of accompanying services.
On average, DATs designed for high-income settings are not prohibitively expensive. There are multiple DATs designed for HICs that sell for roughly $30-40, which is equivalent to products sold into LMICs. This suggests that in the future, DATs could find sustainable markets across country income levels, improving the value proposition for serving TB-affected individuals.
Business models geared for high-income contexts point towards opportunities to target wealthier populations in LMICs. Some DAT companies, such as MedReady, have adopted a direct-to-consumer sales model, while others like Pillsy can be purchased from Amazon.
For consistency, the price ranges represent only physical DAT products and do not account for digital services necessary to use them. These constitute off-the-shelf prices, and should not be used for budgeting purposes. For per-patient costs and budget planning, the ASCENT project has developed a Total Cost of Ownership tool, that covers a number of DATs.